
We recently presented our poster at SITC 2020.
Note: the full text of the abstract is here, download the poster.
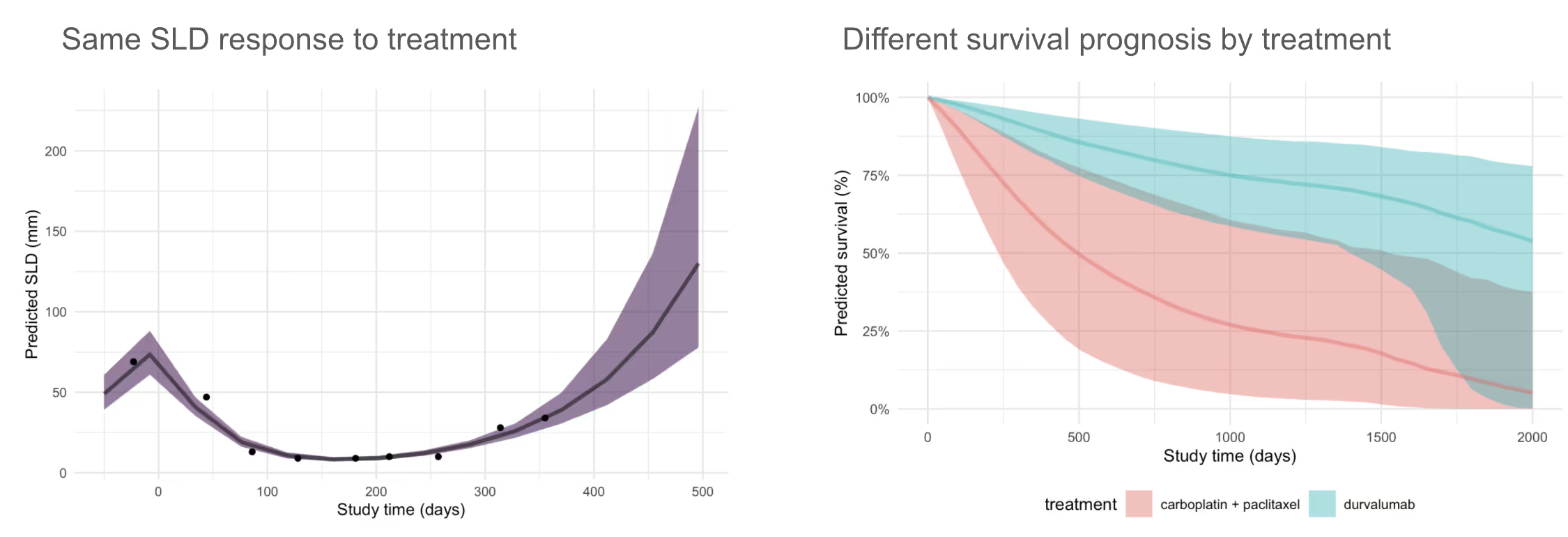
Main findings: Not all reductions in tumor size are equal in Non-Small Cell Lung Cancer (NSCLC).
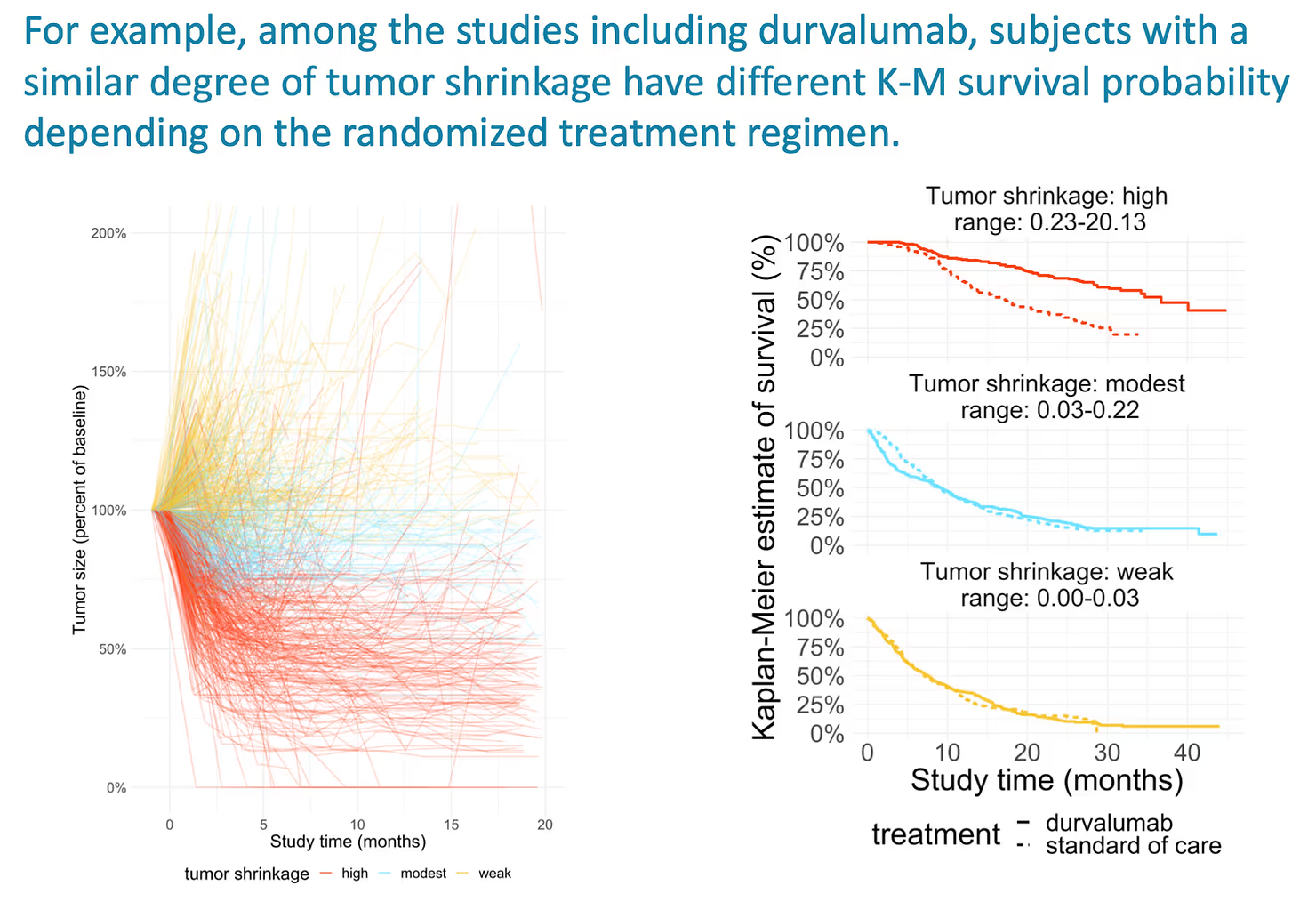
Although we characterized this finding using a Joint Model, it is also evident when plotting Kaplan-Meier survival estimates by category of tumor shrinkage.
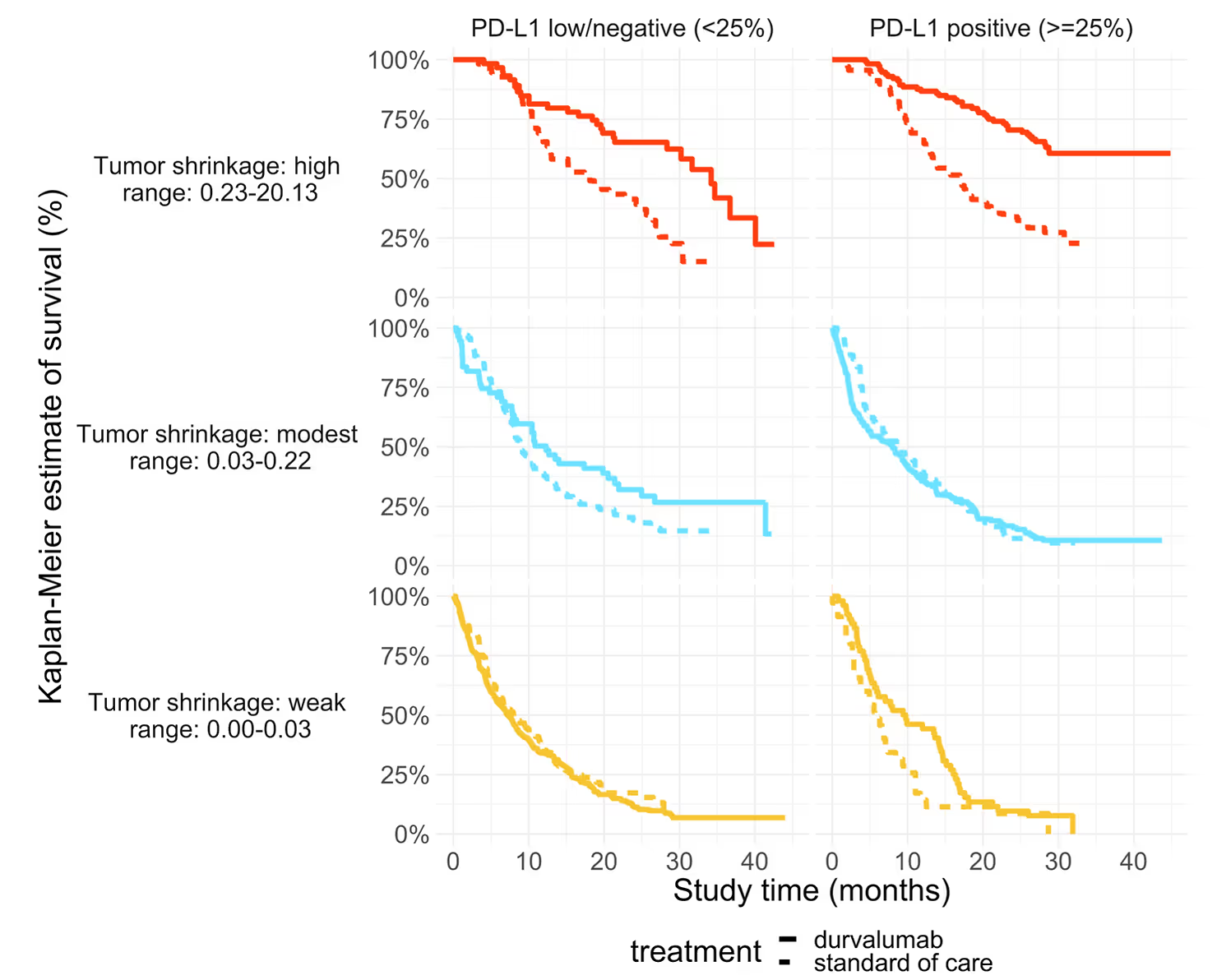
The improved survival benefit for the same tumor shrinkage was observed among subjects with high (>25%) PD-L1 expression and low (<= 25%) PD-L1 expression on tumor cells.
This is from an analysis of patient-level data for over 6,000 subjects across 15 NSCLC clinical trials.
That’s the high-level summary. Read on to learn more about the details.
In solid tumors (and in NSCLC specifically), we use changes in tumor size to test whether a therapy is working. A tumor shrinks when a treatment is working; it grows (or grows back) when it does not. A reduction in tumor size, even off-treatment, is good news. Patients with tumor shrinkage will have a better survival prognosis than patients without tumor shrinkage.
The question we have asked is: how much better?
How much better is your survival when your tumor shrinks by a certain amount?
And is the improvement in survival per reduction in tumor size the same across different therapeutic regimens?
To answer these questions, we looked at patient-level data from 15 randomized clinical trials in non-small-cell lung cancer (NSCLC) made available by AstraZeneca Plc. The trial arms include therapies with diverse mechanisms of action: VEGF-inhibitors, MEK inhibitors, PD-1/PD-L1 inhibitors, CTLA-4 inhibitors, and of course chemotherapy. The analysis includes over 6,000 patients. We summarize their characteristics in [Table 1].
We fit a model to the longitudinal tumor-size data. This biomarker model has been described previously [1]. It describes tumor size over time as a mixture-of-exponents, where some fraction (f) of the tumor is shrinking with exponential decay of ks, and a remaining fraction (1−f) is growing with an exponential growth rate of kg.
For each patient, we get posterior estimates of these parameters: f, kg, and ks. From these, we compute an overall measure of “tumor_shrinkage” and a measure of “tumor regrowth” transformed to be on comparable scales.
$$\text{TS}(t) = \text{TS}_0 \times
\left(
\underbrace{\textcolor{green}{f \cdot \exp(-k_s t)}}_{\textcolor{black}{\text{shrinkage: } f \cdot k_s \cdot 100}} +
\underbrace{\textcolor{red}{(1 - f) \cdot \exp(k_g t)}}_{\textcolor{black}{\text{regrowth: } (1 - f) \cdot k_g \cdot 1000}}
\right)$$
These two quantities are not correlated to one another, and together, they characterize the main features of a tumor-size trajectory.
We then incorporate these two measures (tumor shrinkage & tumor regrowth) into a Cox PH survival model as patient-level covariates. This is how we measure the improvement in survival per reduction in tumor size and the increased risk of mortality per unit change in tumor regrowth.
$$ h_i(t) = h_0(t, \omega) \times \exp\left( \beta_1 \textcolor{green}{\text{shrinkage}_i} + \beta_2 \textcolor{red}{\text{regrowth}_i} \right) $$
The two models – the survival and the biomarker model – are fit jointly (simultaneously) using Bayesian inference. This ensures that all the uncertainty from the biomarker model is incorporated into the survival model. However, this process can be (and sometimes is) done using two separate steps.
We find that patients with more tumor shrinkage do, indeed, have improved survival prognosis than patients with less shrinkage. And, patients with more tumor regrowth have worse overall survival.
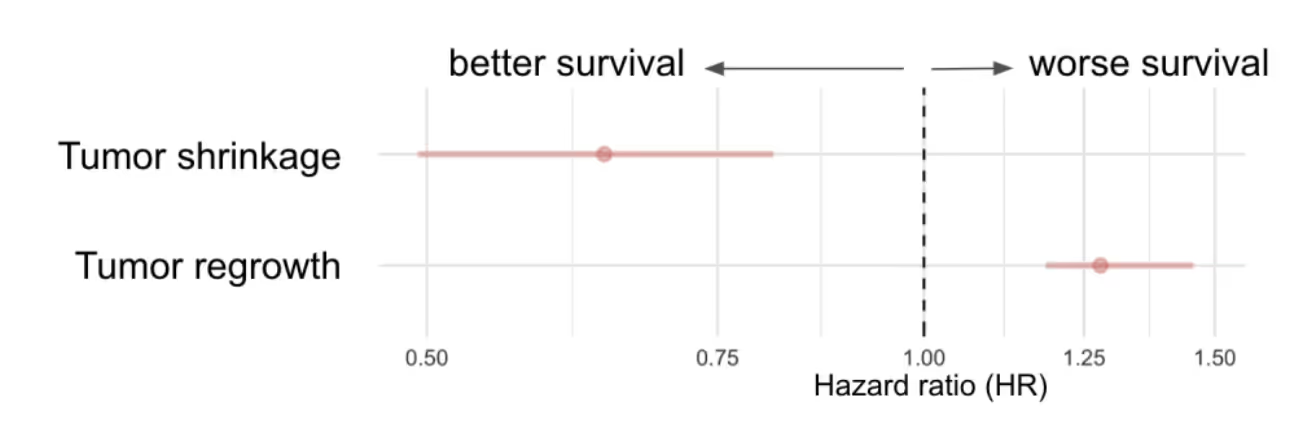
This, in itself, is not surprising. We know from decades of clinical experience that reductions in tumor size correspond to better overall survival, and that tumor regrowth corresponds to worse survival. What is possibly surprising is the magnitude of this effect. In this analysis, the two derived quantities from the tumor-size data are sufficient to recover the treatment effects for each trial and cohort, with one exception: the immunotherapies.
We then include an interaction term, allowing the association of tumor shrinkage & regrowth with survival to vary by treatment class. The results were very interesting. The improvement in survival per unit of tumor shrinkage was much greater among patients receiving a PD-1/PD-L1 inhibitor than among patients receiving other classes of treatments.
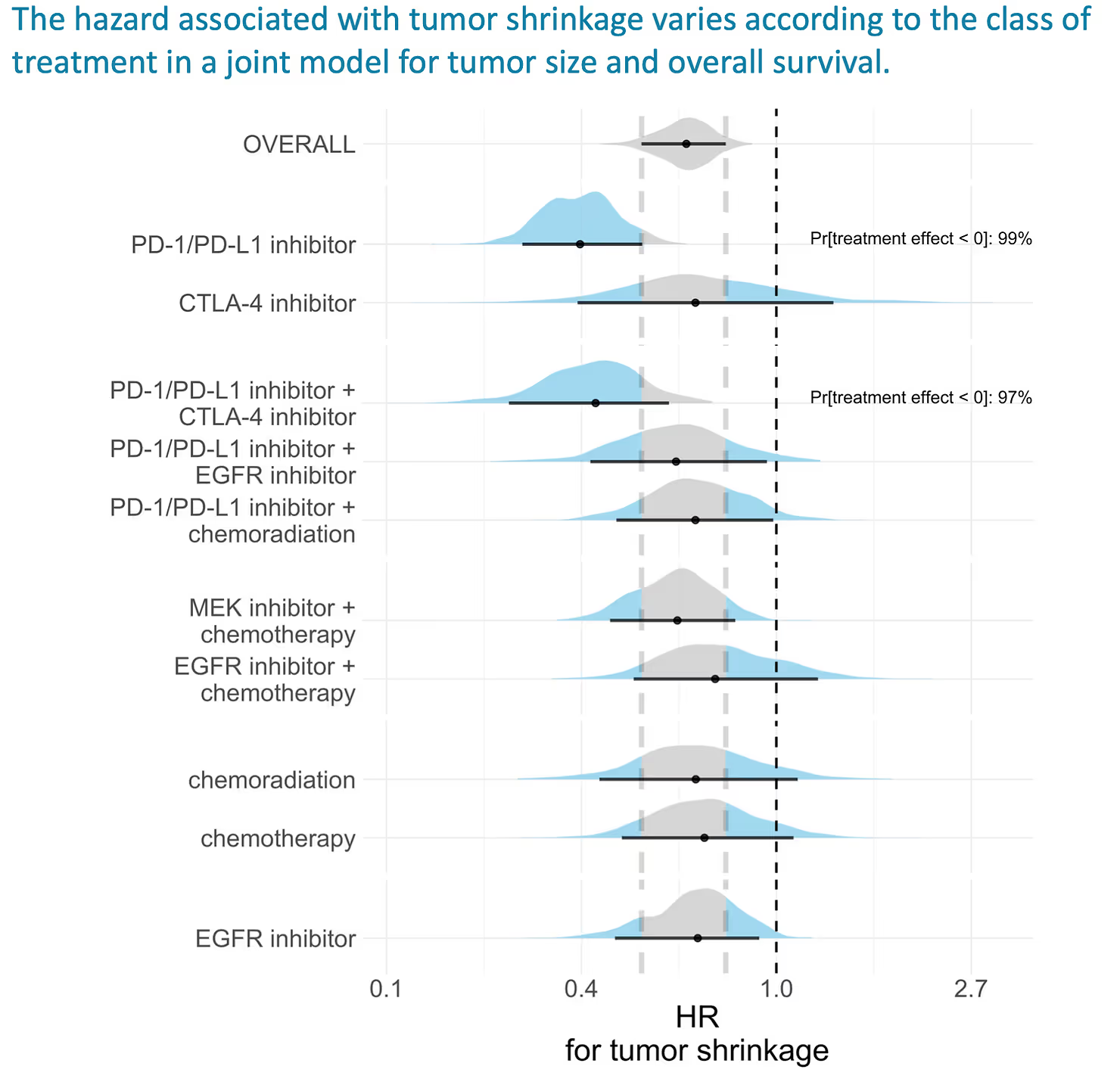
If we assume momentarily a causal relationship among these features, we would say that a patient on a PD-1/PD-L1 inhibitor with a particular amount of tumor shrinkage has a much greater reduction in mortality than a patient with the same tumor shrinkage receiving another class of therapy.
The magnitude of this difference was also quite large, analogous to a treatment effect with an HR of 0.4. In this plot, we predict the overall survival for the same tumor size data for a particular patient under two different treatment scenarios:
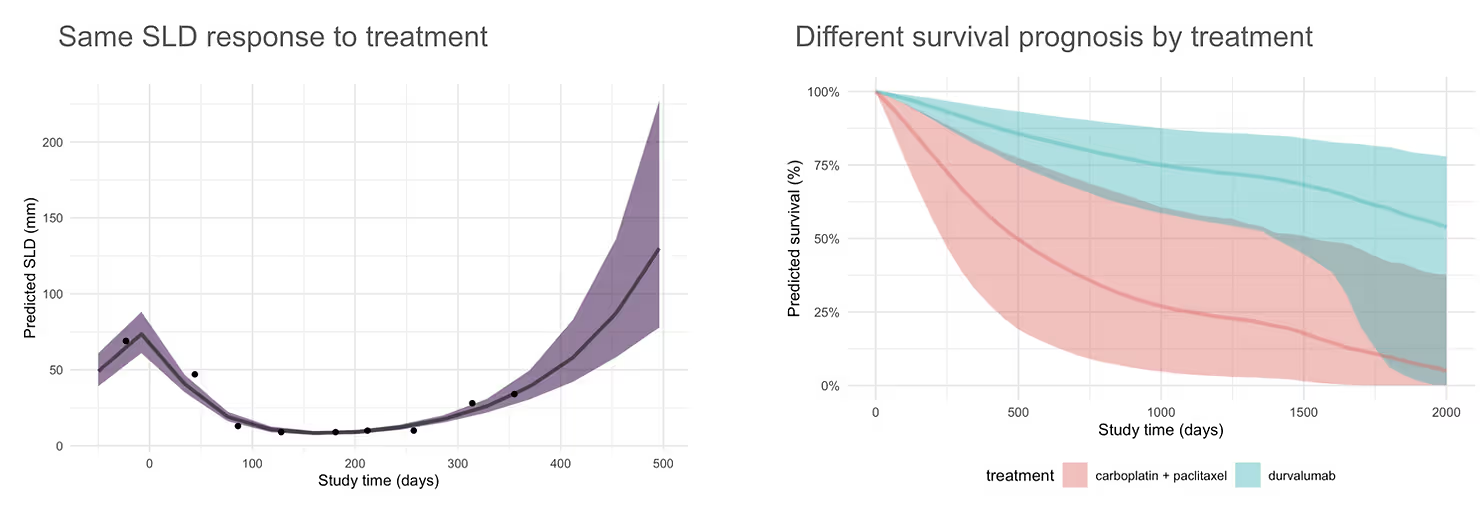
The magnitude of this effect was quite shocking, so we looked back at the source data to substantiate it. We first segmented patients into those with high, medium, and low tumor shrinkage (using cut points at the 33rd and 66th quantile values across the full set of 15 trials to define tertiles of tumor shrinkage).
We then plotted the Kaplan-Meier curves for observed overall survival in each subgroup of patients for the 3 studies that evaluated a PD-1/PD-L1 inhibitor vs standard-of-care control.

This surprised me the first time I saw it. Even among patients with high tumor shrinkage, the survival is much better for patients receiving the PD-1/PD-L1 inhibitor than those on SOC. These patients show evidence of a clinical benefit that is beyond what we expect given the observed tumor shrinkage. An “effect multiplier”, so to speak.
This result suggests that some part of the improved OS for PD-1/PD-L1 inhibitors is due to the rate of response (the portion of patients with tumor shrinkage), and that a substantial part is a function of improved OS among responders.
We then split the cohorts according to PD-L1 expression on tumor cells (> 25% vs <25%), to see if the “effect multiplier” is independent of the assessed level of expression.

Indeed, it was.
Quoting from our poster:
This analysis was a collaborative effort between Generable and AstraZeneca.
The team working on this project included:
The model itself builds on work completed during an earlier collaboration with Sam Brilleman.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.

Weighing pros and cons of NCA vs. PK modeling.

This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.