
This week, I attended the Cardiovascular Clinical Trialists Forum (CVCT) 2025 conference in Washington, DC for the first time. As the name suggests, this conference brings together clinicians and clinical researchers working in cardiometabolic diseases to discuss their methods and trials. There was a small statistics track and even a short Bayesian talk by Scott Barry. AI was everywhere, with the human-in-the-loop theme dominating, for now.
Our work was represented by C Michael Gibson, MD (see Acknowledgement section for other contributors), who was one of our collaborators and the lead author on a paper that was simultaneously published in JACC Advances [1]. We debated the title of the paper with the co-authors and finally settled on "Bayesian machine learning model guiding iterative, personalized anticoagulant dosing decision-making : ENGAGE AF-TIMI 48 trial analysis." Machine learning here should be read as anything numerically inferential is a kind of machine learning, not that we used any non-parametric methods like Neural Nets, deep or otherwise.
We start with the problem statement.
Modern clinical practice relies on evidence from population-based trials, requiring physicians to apply these findings to treat individual patients. However, population-level results often fail to capture critical nuances at the patient level: interaction of multiple complex risk factors, changes in patient characteristics including intercurrent events during follow-up, and individual patient benefit–risk preferences. Furthermore, regulatory-approved dosing recommendations reflect efficacy-safety trade-offs optimized for study population averages rather than individual patients.
This highlights one of the fundamental issues facing clinicians who are trying to implement study results in their practice: the decision to approve the drug and the decision to prescribe the drug are two different things, and somewhat counterintuitively this is a good thing, as argued by Frank Harrell in "Randomized Clinical Trials Do Not Mimic Clinical Practice, Thank Goodness" [2].
The paper is written for clinical researchers, not statisticians, and so important technical details and simulations are reserved for a separate manuscript, which we hope to publish in 2026. For those who want a preview, Jacki wrote a short technical explainer of the hazard submodel in her post "Improving dosing decisions."
During Edoxoban trial ENGAGE AF-TIMI 48 [3], on which the model was based, the researchers collected drug concentration data (pre- and post) as well as clinical events, which made it particularly well suited for estimating the influence of drug exposure on cardiovascular events. To make things concrete, take a look at the following cartoon of the model. We are estimating one large model using the dynamic HMC algorithm in Stan, consisting of the exposure and hazard submodels, where the mean exposure from the PK submodel informs the hazard rate in the multi-state hazard submodel.
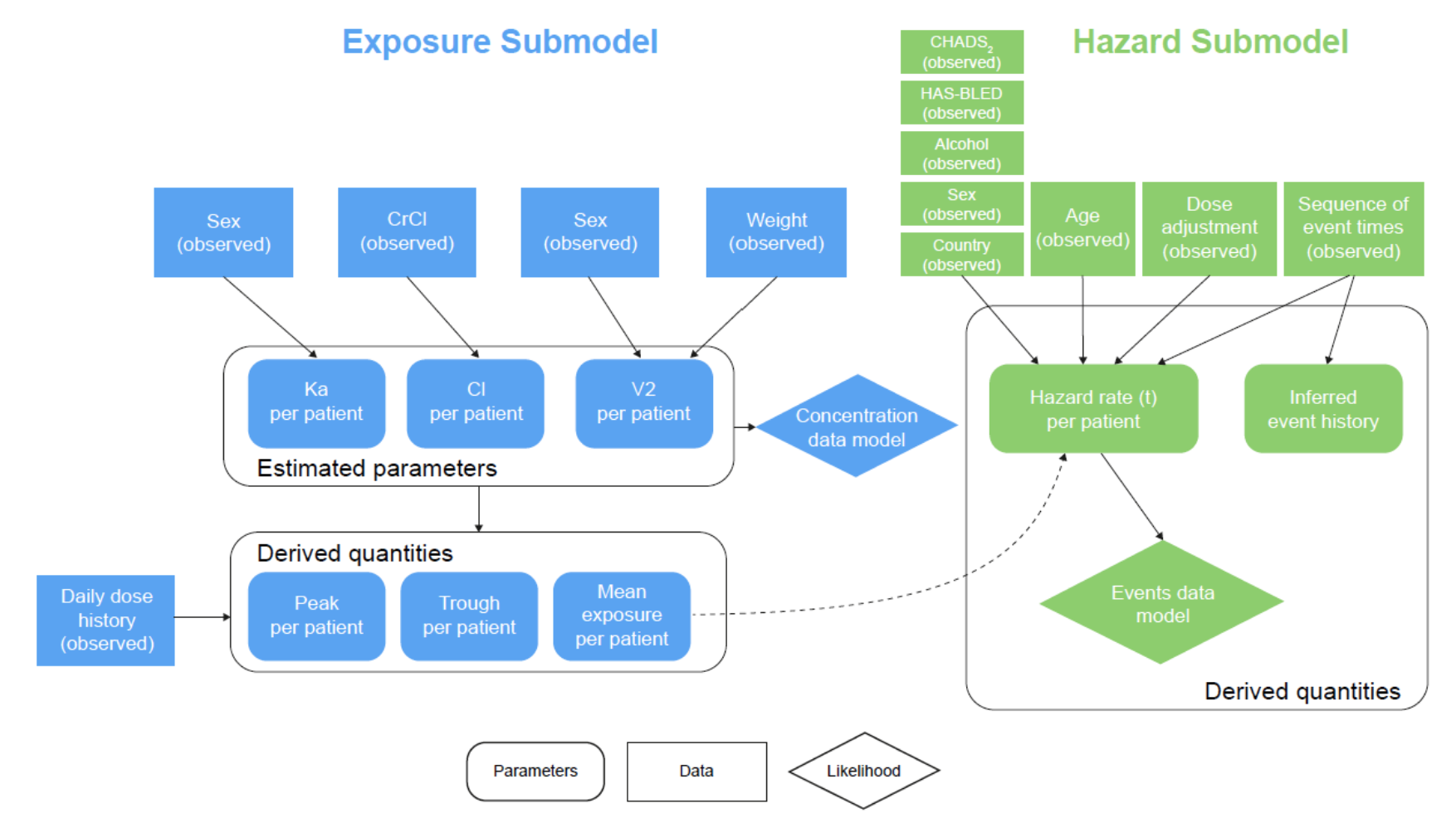
This structure allows us to make better event predictions than using dose alone. A more subtle benefit is that, during routine clinical care, when physicians don't measure drug concentrations, the model first enables us to predict drug exposure given a hypothetical dose and covariates, and then to predict event rates.
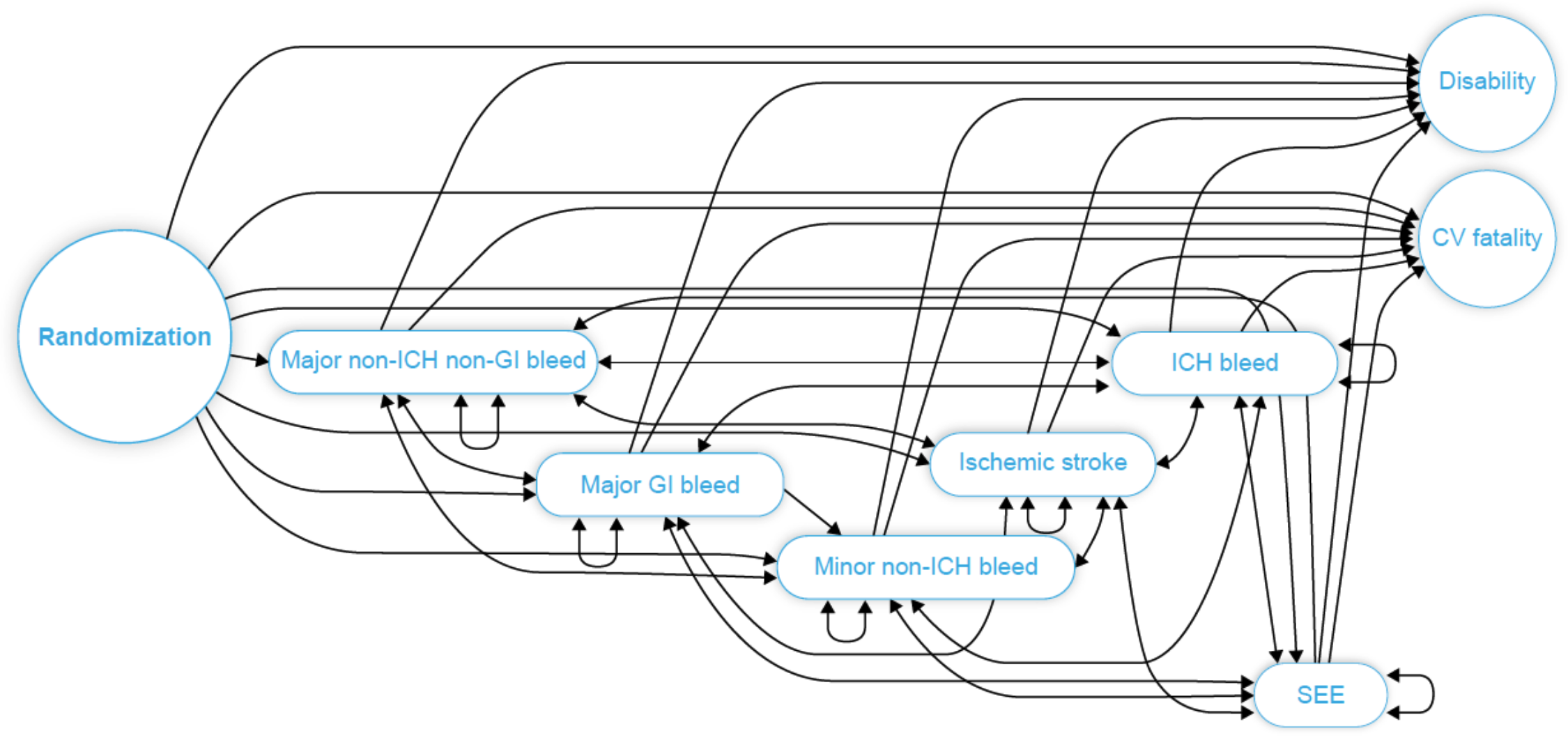
Traditional RCT analysis is usually conducted using a composite endpoint, such as MACE (Major Adverse Cardiovascular Events), which makes it impossible to differentiate among different types of risk. Instead, our hazard model is multi-state, which can be represented as a state transition graph below.

Each full path (one or more transitions) on the graph from randomization to censor (not shown), disability, or CV fatality, represents a possible patient's journey, with self-loops denoting repeating events. This structure enables us to perform a dynamic risk assessment using conditional event rates at different points in time. For example, a patient who has experienced an ischemic stroke is at a higher risk of CV fatality than one who has had, say, a minor non-ICH bleed.
Finally, we address the issue with the notion that a clinician always knows the best course of action for the patient. Different people, quite rationally, can have different risk preferences, and those preferences should be taken into account when making treatment decisions. In decision theory literature, which is quite extensive in statistics and economics, and less so in biomedicine, risk preferences are quantified using a utility function or a loss function, which is just negative utility. Eliciting those and other uncertain quantities from people is not an easy feat, but there is some research in this area as well; see, for example, O’Hagan et al. [4].
In the paper, we did not conduct any studies to elicit patient preferences. Instead, we demonstrate how the model can be used under a hypothetical loss function, which is a linear weighted sum of the predicted event rates for five types of cardiovascular events with weights supplied by the user. The dose recommendation, which corresponds to the lowest predicted 3-year event rate, is computed by averaging the loss function over all predicted event probabilities (i.e., computing the expected loss), following the standard method of Bayesian decision analysis, as described, for example, in Chapter 9 of Gelman et al. [5].
The graph below demonstrates this by considering a hypothetical patient who wants to avoid major bleeding more than CV death, with the risk preference specified in the upper right, and the predicted event rates for each dose.
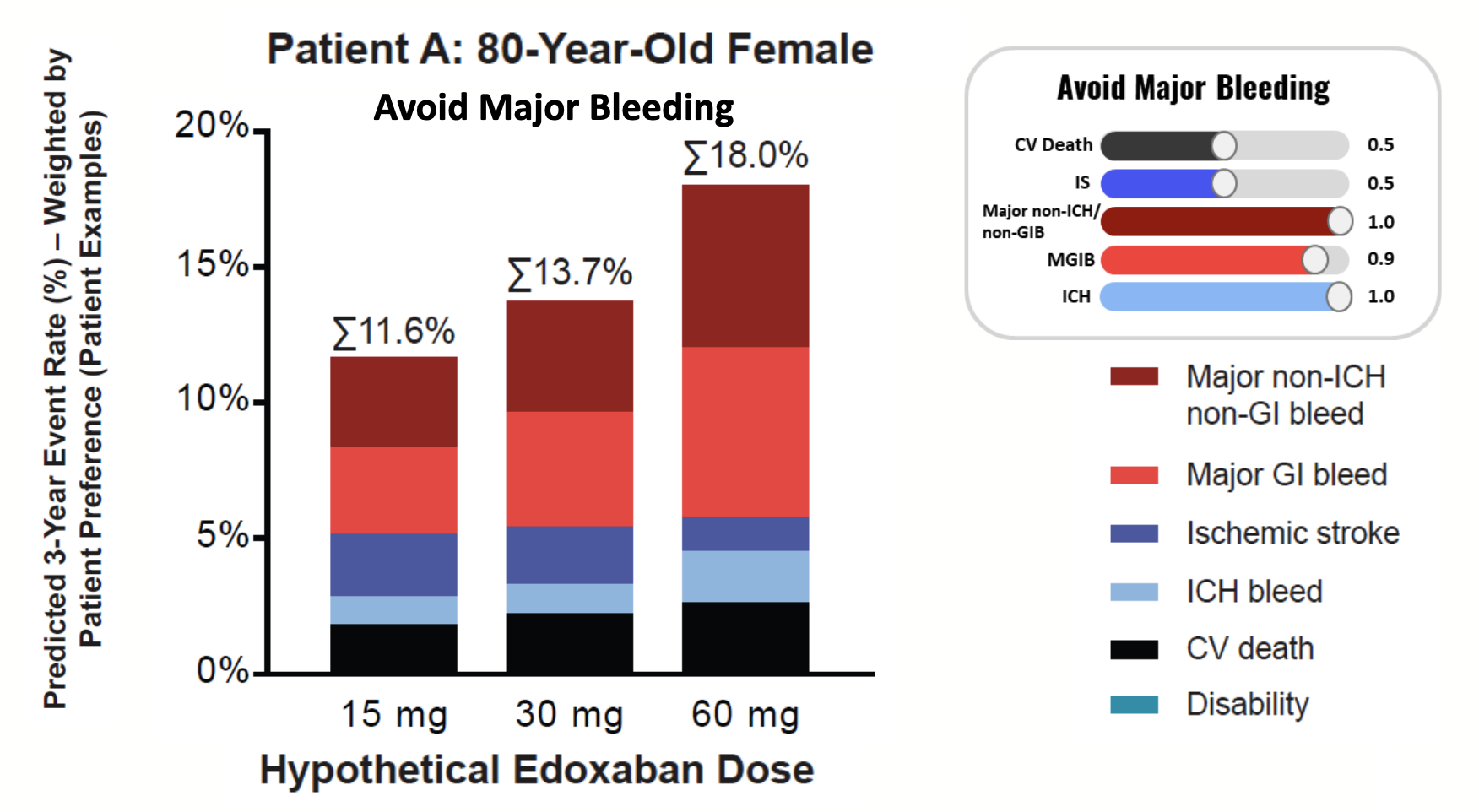
In this case, the lowest loss is achieved with a 15mg dose, with a total expected event rate of about 11.6%. This makes sense, since anticoagulants induce bleeding, and so the lowest dose should minimize this effect. The CV death rate is also slightly reduced, but this is offset by a large increase in Ischemic stroke compared with the highest dose. These are complicated decisions, and we envision that they would be made in consultation with the physician.
To ease comparison of event rates across doses, I created the line graph below. Here, you can clearly see the downward-sloping, dark-blue Ischemic stroke line as a function of dose, further highlighting the trade-offs.
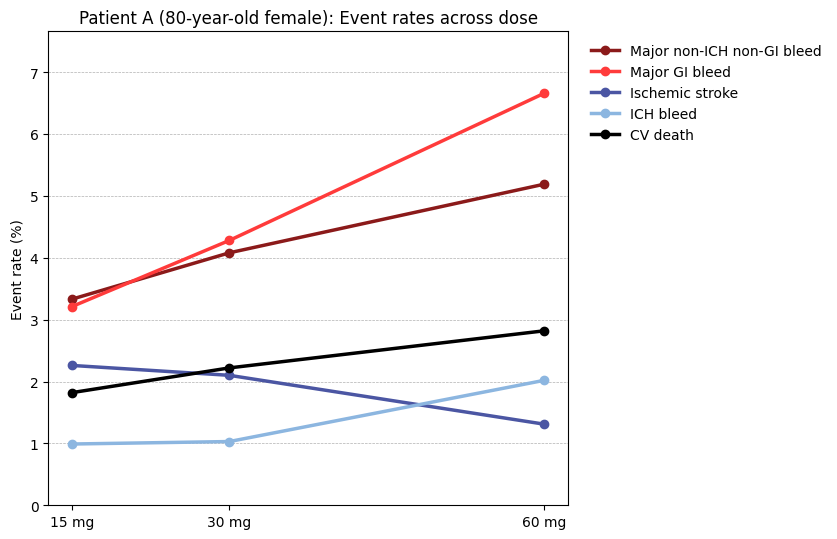
This model was trained on data from the Edoxoban RCT, while patients entering routine clinical care are more diverse and may have other comorbidities that were likely excluded from the trial. As mentioned before, that's not a limitation of the RCT, which tries to get the cleanest measure of the treatment effect. Rather, before assessing how well the model performs in the wild, we should conduct a validation study using a cardiovascular registry with longer follow-up and a more diverse population. It would also be interesting to evaluate this model's performance with other anticoagulants, particularly those with a different mechanism of action.
This project, code-named Adele, was a large collaboration and we would like to thank the following individuals for their contribution.
• C. Michael Gibson - Baim Institute for Clinical Research, Harvard Medical School, Boston, Massachusetts, USA
• Cathy Chen - Daiichi Sankyo, Inc., Basking Ridge, New Jersey, USA
• Jacqueline Buros-Novik - Generable, New York, New York, USA
• Juho Timonen - Generable, New York, New York, USA
• Eugene Braunwald - TIMI Study Group, Cardiovascular Division, Brigham and Women’s Hospital, Department of Medicine, Harvard Medical School, Boston, Massachusetts, USA
• Elliott M. Antman - TIMI Study Group, Cardiovascular Division, Brigham and Women’s Hospital, Department of Medicine, Harvard Medical School, Boston, Massachusetts, USA
• Eva-Maria Fronk - DSS-EG, Daiichi Sankyo Europe GmbH, Munich, Germany
• Matthew Clasen - Daiichi Sankyo, Inc., Basking Ridge, New Jersey, USA
• Robert P. Giugliano - TIMI Study Group, Cardiovascular Division, Brigham and Women’s Hospital, Department of Medicine, Harvard Medical School, Boston, Massachusetts, USA
• Martin Unverdorben - Daiichi Sankyo, Inc., Basking Ridge, New Jersey, USA
[1] Gibson, C. M., Chen, C., Buros-Novik, J., Novik, E., Timonen, J., Braunwald, E., Antman, E. M., Fronk, E.-M., Clasen, M., Giugliano, R. P., & Unverdorben, M. (2025). Bayesian machine learning model guiding iterative, personalized anticoagulant dosing decision-making : ENGAGE AF-TIMI 48 trial analysis. JACC Advances, 102504.
[2] Harrell, F. (2023, February 14). Randomized Clinical Trials Do Not Mimic Clinical Practice, Thank Goodness. Statistical Thinking. https://www.fharrell.com/post/rct-mimic/
[3] Giugliano, R. P., Ruff, C. T., Braunwald, E., Murphy, S. A., Wiviott, S. D., Halperin, J. L., Waldo, A. L., Ezekowitz, M. D., Weitz, J. I., Špinar, J., Ruzyllo, W., Ruda, M., Koretsune, Y., Betcher, J., Shi, M., Grip, L. T., Patel, S. P., Patel, I., Hanyok, J. J., … ENGAGE AF-TIMI 48 Investigators. (2013). Edoxaban versus warfarin in patients with atrial fibrillation. The New England Journal of Medicine, 369(22), 2093–2104.
[4] O’Hagan, A., Buck, C. E., Daneshkhah, A., Eiser, J. R., Garthwaite, P. H., Jenkinson, D. J., Oakley, J. E., & Rakow, T. (2006). Uncertain judgements: Eliciting experts’ probabilities. Wiley-Blackwell.
[5] Gelman, A., Carlin, J. B., Stern, H. S., Dunson, D. B., Vehtari, A., & Rubin, D. B. (2013). Bayesian data analysis, (3rd ed.). Chapman & Hall/CRC. https://doi.org/10.1007/s13398-014-0173-7.2
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.
From R/Stan to Julia/Turing

This is a comment related to the post above. It was submitted in a form, formatted by Make, and then approved by an admin. After getting approved, it was sent to Webflow and stored in a rich text field.